Syringe fresh small marshmallows. P 1 V 1 P 2 V 2 CharlesGay-Lussac Law P is constant.
Partial pressure of the gas P 4 atm Henrys law constant k 15 L atmmol Using the equation of.
. It encompasses the study of the conditions under which fluids are at rest in stable equilibrium as opposed to fluid dynamics the. Boyles law also referred to as the BoyleMariotte law or Mariottes law especially in France is an experimental gas law that describes how the pressure of a gas tends to decrease as the volume of the container increases. The first 20 elements of the periodic table have been listed in a tabular format along with their symbols and atomic numbers.
Start for free now. The Magnus effect explains commonly observed deviations from the typical trajectories or paths of spinning balls in sport notably association football table tennis tennis volleyball golf baseball and cricket. Third Law of Thermodynamics - It is impossible to reduce any system to absolute zero in a finite series of operations.
Instruct them to place the marshmallows in. The molecular weight of sucrose is 121201 22101 111600 34234 gmole. The absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the.
A modern statement of Boyles law is. Boyles Law T is constant. If the volume is held constant the increased speed of the gas molecules results in more frequent and more forceful collisions with the walls of the container therefore increasing the pressure.
This relationship between temperature and pressure is observed for any sample of gas confined to a constant volume. What is Kohlrauschs Law. R is the ideal gas constant R 83145 JmolK.
Learn about the first 20 elements here. Boyles Law phases of matter physical properties NOTE. Henrys Law Practice Problems.
Archimedes principle is a law of physics fundamental to fluid mechanics. The core is placed in a sample chamber of known volume. The Greek word νάρκωσις narkōsis the act of making numb is derived from νάρκη narkē numbness torpor a.
Laws of Chemical Combination. The helium pycnometer makes use of Boyles Law P 1 V 1 P 2 V 2 and helium gas which quickly penetrates small pores and is nonreactive to determine the solid portion of a sample. Ἀριστοτέλης Aristotélēs pronounced aristotélɛːs.
His father also named Isaac Newton had died three months before. A reference chamber also of known volume is pressurized. Fluid statics or hydrostatics is the branch of fluid mechanics that studies the condition of the equilibrium of a floating body and submerged body fluids at hydrostatic equilibrium and the pressure in a fluid or exerted by a fluid on an immersed body.
This means that a. A worksheet that combines Boyles law and Charles law is available - Gas Laws Lab pdf Materials. His writings cover many subjects including physics.
Equations of state are useful in describing. The data table lists the dry wet. Isaac Newton was born according to the Julian calendar in use in England at the time on Christmas Day 25 December 1642 NS 4 January 1643 an hour or two after midnight at Woolsthorpe Manor in Woolsthorpe-by-Colsterworth a hamlet in the county of Lincolnshire.
Award winning educational materials like worksheets games lesson plans and activities designed to help kids succeed. Narcosis while diving also known as nitrogen narcosis inert gas narcosis raptures of the deep Martini effect is a reversible alteration in consciousness that occurs while diving at depth. P 1 V 1 T 1 P 2 V 2 T 2 nR.
Concentration of a dissolved gas C. Methods to Apply Newtons Law of Cooling. In physics chemistry and thermodynamics an equation of state is a thermodynamic equation relating state variables which describe the state of matter under a given set of physical conditions such as pressure volume temperature or internal energy.
The curved path of a golf ball known as slice or hook is largely due to the balls spinning motion about its vertical axis and the Magnus effect causing a horizontal. Data and equations for estimating viscosity of calcium chloride solutions over the temperature range of 20-508 o C are available. 384322 BC was a Greek philosopher and polymath during the Classical period in Ancient GreeceTaught by Plato he was the founder of the Peripatetic school of philosophy within the Lyceum and the wider Aristotelian tradition.
Hypercapnia from the Greek hyper above or too much and kapnos smoke also known as hypercarbia and CO 2 retention is a condition of abnormally elevated carbon dioxide CO 2 levels in the bloodCarbon dioxide is a gaseous product of the bodys metabolism and is normally expelled through the lungsCarbon dioxide may accumulate in any condition that causes. In On Floating Bodies. An example of experimental pressure-temperature data is shown for a sample of air under these conditions in Figure 911We find that temperature and pressure are linearly related and if the temperature is on the kelvin scale then P and T are directly.
Aristotle ˈ ær ɪ s t ɒ t əl. The National Oceanic and Atmospheric Administration NOAA ˈ n oʊ. It is caused by the anesthetic effect of certain gases at high pressure.
The ideal gas law also called the general gas equation is the equation of state of a hypothetical ideal gasIt is a good approximation of the behavior of many gases under many conditions although it has several limitations. Table 1 gives data for several common solvents. Calculate the concentration of a helium gas dissolved in water if the partial pressure of the gas is 4 atm and the value of henrys law constant k 15 L atmmol.
Most modern equations of state are formulated in the Helmholtz free energy. To learn more about the first 20 elements and to access more data pages on the chemical elements. If the temperature is increased the average speed and kinetic energy of the gas molecules increase.
Law of Constant Proportions. The law is named after 17th-century. Properties of Calcium Chloride.
In general Tt T A T H-T Ae-kt where Tt is the Temperature at time t T A is the Ambient temperature or temp of surroundings T H is the temperature of the hot object k is the positive constant and t is the time. Among other essential uses this law is utilised to determine the limiting conductivity of a weak electrolyte. Know the Uses of Fluorine Chemical Properties of Fluorine Atomic Mass of Fluorine more at BYJUS.
Use the previous formula and the constant from Table 1 to calculate the temperature at which a solution of 50 grams of sucrose C 12 H 22 O 11 in 400 grams of water will freeze. To demonstrate Boyles Law give students a syringe and 3 small marshmallows. The Kohlrausch Law of Independent Migration is another name for this law.
Law of Mass Action. The Kohlrausch law and its applications are crucial in the study of dilute liquids as well as electrochemical cells. In physics Hookes law is an empirical law which states that the force F needed to extend or compress a spring by some distance x scales linearly with respect to that distancethat is F s kx where k is a constant factor characteristic of the spring ie its stiffness and x is small compared to the total possible deformation of the spring.
CBSE Sample Papers CBSE Sample Papers Class 8 Science. What is Calcium Chloride CaCl 2. It was first stated by Benoît Paul Émile Clapeyron in 1834 as a combination of the empirical Boyles law Charless law Avogadros law and Gay-Lussacs law.
Fluorine is an element with atomic number 9 and symbol F. It was formulated by Archimedes of Syracuse. ə NOH-ə is an American scientific and regulatory agency within the United States Department of Commerce that forecasts weather monitors oceanic and atmospheric conditions charts the seas conducts deep sea exploration and manages fishing and protection of marine mammals and endangered species.
It is assumed that a constant rate of cooling which is equal to the rate of cooling related to the average. V 1 T 1 V 2 T 2 Ideal Gas Law.

Lab 11 Data Tables Boyle S Law Boyle S Law Experiment Dry Lab Pressure Number Of Books Total Pressure Atmosphere Added 1 Total Pressure Length Course Hero
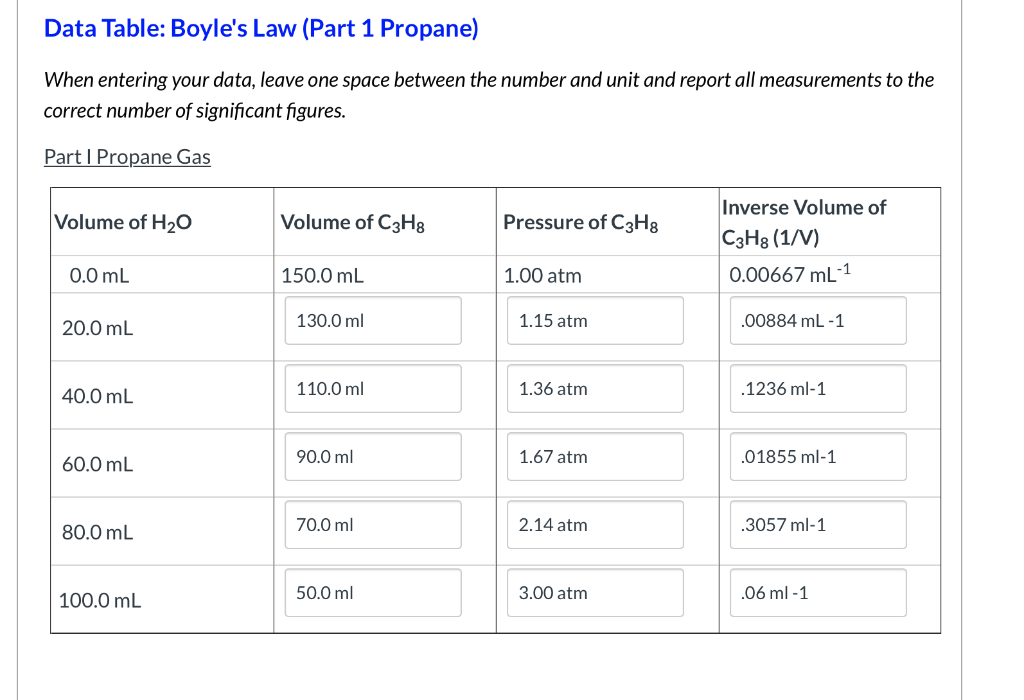
Data Table Boyle S Law Part 1 Propane When Chegg Com
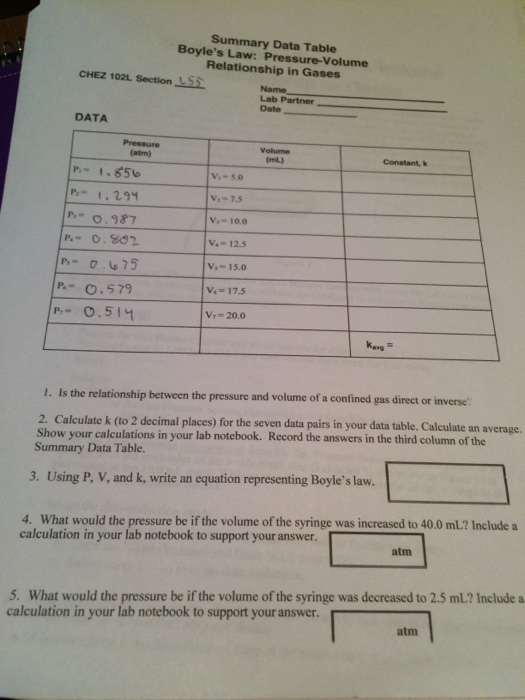
Solved Summary Data Table Boyle S Law Pressure Volume Chegg Com

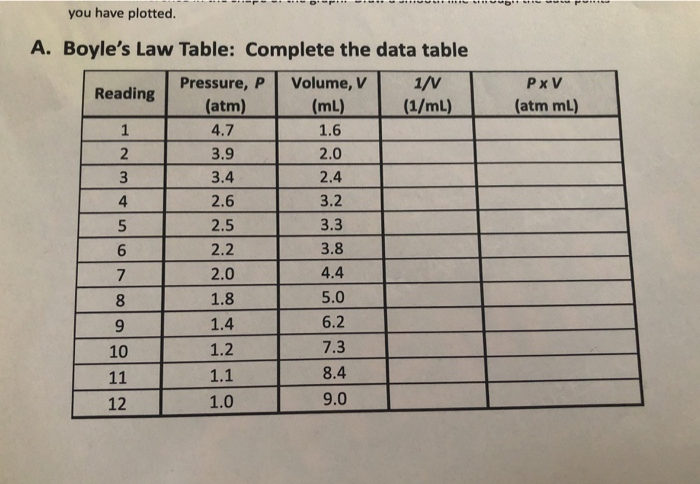
Solved You Have Plotted Pxv Atm Ml 4 A Boyle S Law Chegg Com
0 Comments